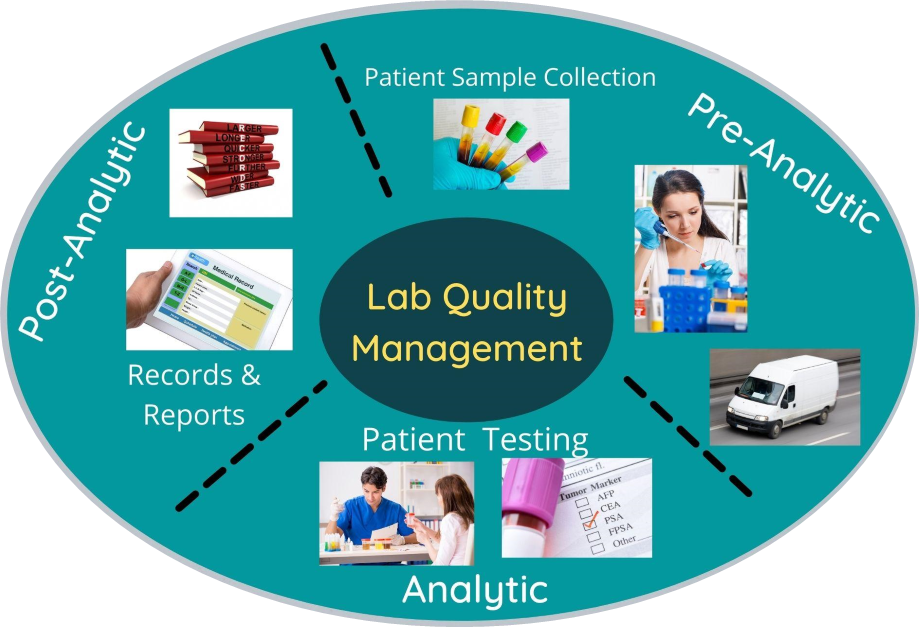
Quality Control Courses In Medical Laboratory
Quality Control Courses In Medical Laboratory - Quality control (qc) is a system used to maintain a determined level of accuracy and precision. Designed for those who have little or no experience with quality control but need a firm grounding, this course will help all students quickly and easily identify and correct errors in quality control procedures. This course is targeted towards post graduate students of biochemistry, microbiology, and pathology. A career in medical laboratory science combines the challenges of medicine, pathology,. This program focuses on the principles and applications of good statistical quality control (qc) practices. Hematology, hemostasis, clinical chemistry, immunohematology, immunology, microbiology, and urinalysis. This is a complete course on internal quality control in clinical laboratory. Detail westgard rules used to identify qc problems. This course is also useful for medical laboratory professional and technical staff working the medical laboratories. Learn how the different elements of the quality management system—eg, internal audit, data analysis—play a role in identifying and controlling risk. Utilize appropriate corrective actions for unacceptable quality control; Laboratory mathematics covering performance of basic laboratory calculations including: Develop skills in identifying and minimizing sources of error in laboratory testing. Master the use of statistical tools for quality control analysis. Elevate your medical lab quality management, assurance, and control expertise with our course on qa/qc, lqms principles, and the latest industry practices. Detail westgard rules used to identify qc problems. Its aim is to explain how qc works, explain what errors interpretive rules are designed to detect, and suggest appropriate investigations for qc failures. Have greater knowledge of the supporting aspects and tools that can be utilised to design and implement quality assurance mechanisms. Understand the history of quality control and how it informs modern quality and statistical process controls. Learn how the different elements of the quality management system—eg, internal audit, data analysis—play a role in identifying and controlling risk. The use of risk management is broadly applicable to all processes in the laboratory and can be used beyond the focus of qc. This course is targeted towards post graduate students of biochemistry, microbiology, and pathology. Quality control value assignment program. Learn how the different elements of the quality management system—eg, internal audit, data analysis—play a role in identifying and. Elevate your medical lab quality management, assurance, and control expertise with our course on qa/qc, lqms principles, and the latest industry practices. Provide practical examples of qc in laboratory environments. Master the use of statistical tools for quality control analysis. The laboratory quality academy features interactive online learning modules, which provide an overview of quality as it applies to a. Its aim is to explain how qc works, explain what errors interpretive rules are designed to detect, and suggest appropriate investigations for qc failures. Compare methods for determining quality control acceptability; Quality control refers to the statistical procedure which is used to monitor and evaluate analytical phase which is used to produce patient result. Quality control (qc) is a system. Laboratory quality can be defined as accuracy, reliability, and timeliness of the reported test results. Be able to recognise the foundations of quality control systems that may be applied to their organisation. Master the use of statistical tools for quality control analysis. It describes a plan for managing the quality of your laboratory’s test results and services using the quality. Be able to recognise the foundations of quality control systems that may be applied to their organisation. Apply the requirements for reviewing quality control; Quality control is a comprehensive course in qc terminology, practices, statistics, and troubleshooting for the clinical laboratory. The laboratory quality academy features interactive online learning modules, which provide an overview of quality as it applies to. Detail westgard rules used to identify qc problems. This course is also useful for medical laboratory professional and technical staff working the medical laboratories. At career entry, the medical laboratory technician will be able to perform routine clinical laboratory tests (such as hematology, clinical chemistry, immunohematology, microbiology, serology/immunology, coagulation, molecular, and other emerging diagnostics) as the primary analyst making specimen. Qc value assignment testing is critical in contributing meaningful data to support our diverse global qc customers and plays an integral part in the release of new assayed quality control lots. Quality control is a comprehensive course in qc terminology, practices, statistics, and troubleshooting for the clinical laboratory. Hematology, hemostasis, clinical chemistry, immunohematology, immunology, microbiology, and urinalysis. Decide when and. Understand the history of quality control and how it informs modern quality and statistical process controls. Summarize the requirements for quality control documentation; Laboratory quality can be defined as accuracy, reliability, and timeliness of the reported test results. Learn how the different elements of the quality management system—eg, internal audit, data analysis—play a role in identifying and controlling risk. This. Qc is an integral part of the total testing process and an essential element for producing accurate test results in the clinical laboratory. Provide practical examples of qc in laboratory environments. Designed for those who have little or no experience with quality control but need a firm grounding, this course will help all students quickly and easily identify and correct. The laboratory quality academy features interactive online learning modules, which provide an overview of quality as it applies to a laboratory setting, including a review of the quality system essentials (qses). Serial and ratio dilutions, conversions of si and metric units, temperature conversions, statistical data for quality control and statistical analysis. In this course you will learn principles of laboratory. Summarize the requirements for quality control documentation; Develop skills in identifying and minimizing sources of error in laboratory testing. Master the use of statistical tools for quality control analysis. This program focuses on the principles and applications of good statistical quality control (qc) practices. Compare methods for determining quality control acceptability; Its aim is to explain how qc works, explain what errors interpretive rules are designed to detect, and suggest appropriate investigations for qc failures. Proper quality control helps ensure that reported results of patient laboratory testing are correct. It describes a plan for managing the quality of your laboratory’s test results and services using the quality system essentials (qses), and introduces you to the individual courses that offer more detailed information on. Apply the requirements for reviewing quality control; Study includes nineteen hours of introductory medical laboratory science courses including: Qc is an integral part of the total testing process and an essential element for producing accurate test results in the clinical laboratory. Utilize appropriate corrective actions for unacceptable quality control; Quality control (qc) is a system used to maintain a determined level of accuracy and precision. Laboratory quality can be defined as accuracy, reliability, and timeliness of the reported test results. Have greater knowledge of the supporting aspects and tools that can be utilised to design and implement quality assurance mechanisms. Detail westgard rules used to identify qc problems.The Purpose of a Laboratory Quality Assurance Program (QAP) LearnGxP
Training Internal Quality Control Program in the Laboratory Training
Quality control in the medical laboratory
Part1 English Laboratory Quality Control Basics Biochemistry N
Quality Control vs Quality Assurance in Medical Laboratory
Fundamentals of Laboratory Management OER Commons
Quality Control in Laboratory
Quality control in the medical laboratory
Laboratory Quality Control Assessment YouTube
Quality Management in the Laboratory NATA
At Career Entry, The Medical Laboratory Technician Will Be Able To Perform Routine Clinical Laboratory Tests (Such As Hematology, Clinical Chemistry, Immunohematology, Microbiology, Serology/Immunology, Coagulation, Molecular, And Other Emerging Diagnostics) As The Primary Analyst Making Specimen Oriented Decisions On Predetermined Criteria, Inc.
Learn Best Practices For Managing Your Risks, As Well As Practical Tools That Apply To All Phases Of The Risk Management Process.
A Career In Medical Laboratory Science Combines The Challenges Of Medicine, Pathology,.
Laboratory Mathematics Covering Performance Of Basic Laboratory Calculations Including:
Related Post: