Computer System Validation Courses
Computer System Validation Courses - Upon completion of this course you will be able to, understand the importance of computer system validation, the regulations and standards behind csv, recommendations and best practices. Helping you become a certified csv. What is computer system validation? Which systems do you need to validate? Understand how to ensure that software meets requirements and performs as expected. Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. The instructor reviews recent fda. The course will equip you with recommendations and best practices for successful csv implementation, as well as an understanding of the gamp®5 framework and the essential. Help your people gain a fuller understanding of the requirements for computer system validation and explore the methods to comply with them with our fda computer system validation. This course is designed for validation, quality, it, and business personnel responsible for implementing and using regulated computer systems in the pharmaceutical, biotech and. These are some of the questions answered in this live. In this computer system validation training you’ll learn how to integrate risk based supplier evaluation into the computer system validation process. Where does validation fit into your sdlc or salc? Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. Csv training, a subsidiary of validation associates llc, specializes in software validation, cleaning validation, instrument/equipment validation, process validation, documentum,. What is computer system validation? Document your dedication to computer system validation (csv) by earning a professional certification from biopharma institute. Validation master plan and system. Earn a gxp computer system validation certificate. The instructor reviews recent fda. Understand how to ensure that software meets requirements and performs as expected. Csv training, a subsidiary of validation associates llc, specializes in software validation, cleaning validation, instrument/equipment validation, process validation, documentum,. What is computer system validation? Start by gaining fundamental knowledge about computer system validation and maintenance based on the gamp 5 second edition. Upon completion of this course you. Understand concepts such as system categorization,. The instructor reviews recent fda. Understand how to ensure that software meets requirements and performs as expected. Helping you become a certified csv. Where does validation fit into your sdlc or salc? Validation master plan and system. This course is designed for validation, quality, it, and business personnel responsible for implementing and using regulated computer systems in the pharmaceutical, biotech and. Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. Where does validation fit into your sdlc or salc? Understand concepts. Helping you become a certified csv. Help your people gain a fuller understanding of the requirements for computer system validation and explore the methods to comply with them with our fda computer system validation. The course will equip you with recommendations and best practices for successful csv implementation, as well as an understanding of the gamp®5 framework and the essential.. This course is designed for validation, quality, it, and business personnel responsible for implementing and using regulated computer systems in the pharmaceutical, biotech and. Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. Start by gaining fundamental knowledge about computer system validation and maintenance based on the gamp 5. The course will equip you with recommendations and best practices for successful csv implementation, as well as an understanding of the gamp®5 framework and the essential. The instructor reviews recent fda. Which systems do you need to validate? Help your people gain a fuller understanding of the requirements for computer system validation and explore the methods to comply with them. Understand concepts such as system categorization,. Pharmout has been training on computer systems validation since 2008, much has changed recently, surprisingly data integrity remains a hot topic with regulators. Understand how to ensure that software meets requirements and performs as expected. What is computer system validation? Upon completion of this course you will be able to, understand the importance of. The instructor reviews recent fda. What is computer system validation? Which systems do you need to validate? Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. Validation master plan and system. Help your people gain a fuller understanding of the requirements for computer system validation and explore the methods to comply with them with our fda computer system validation. These are some of the questions answered in this live. The instructor reviews recent fda. Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective. This course is designed for validation, quality, it, and business personnel responsible for implementing and using regulated computer systems in the pharmaceutical, biotech and. Helping you become a certified csv. The instructor reviews recent fda. Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. In this computer system validation. Helping you become a certified csv. Csv training, a subsidiary of validation associates llc, specializes in software validation, cleaning validation, instrument/equipment validation, process validation, documentum,. What is computer system validation? Learn verification and validation techniques for software quality assurance. Computer system validation for professionals program includes an introduction to the international principles and regulations behind effective validation and qualification. Earn a gxp computer system validation certificate. Validation master plan and system. Help your people gain a fuller understanding of the requirements for computer system validation and explore the methods to comply with them with our fda computer system validation. Document your dedication to computer system validation (csv) by earning a professional certification from biopharma institute. Where does validation fit into your sdlc or salc? The instructor reviews recent fda. Understand concepts such as system categorization,. These are some of the questions answered in this live. Upon completion of this course you will be able to, understand the importance of computer system validation, the regulations and standards behind csv, recommendations and best practices. The course will equip you with recommendations and best practices for successful csv implementation, as well as an understanding of the gamp®5 framework and the essential. In this computer system validation training you’ll learn how to integrate risk based supplier evaluation into the computer system validation process.Computer System Validation Course AnumTechSystems, Inc.
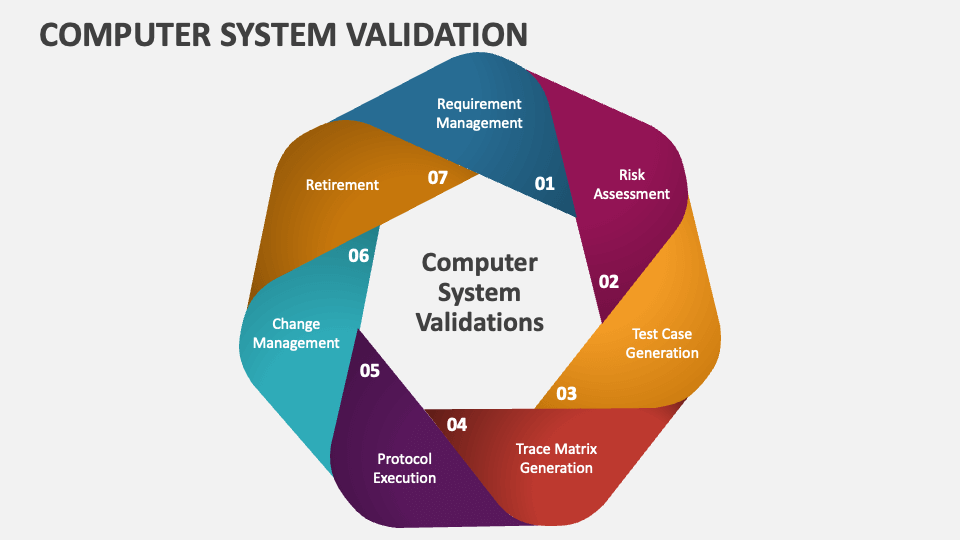
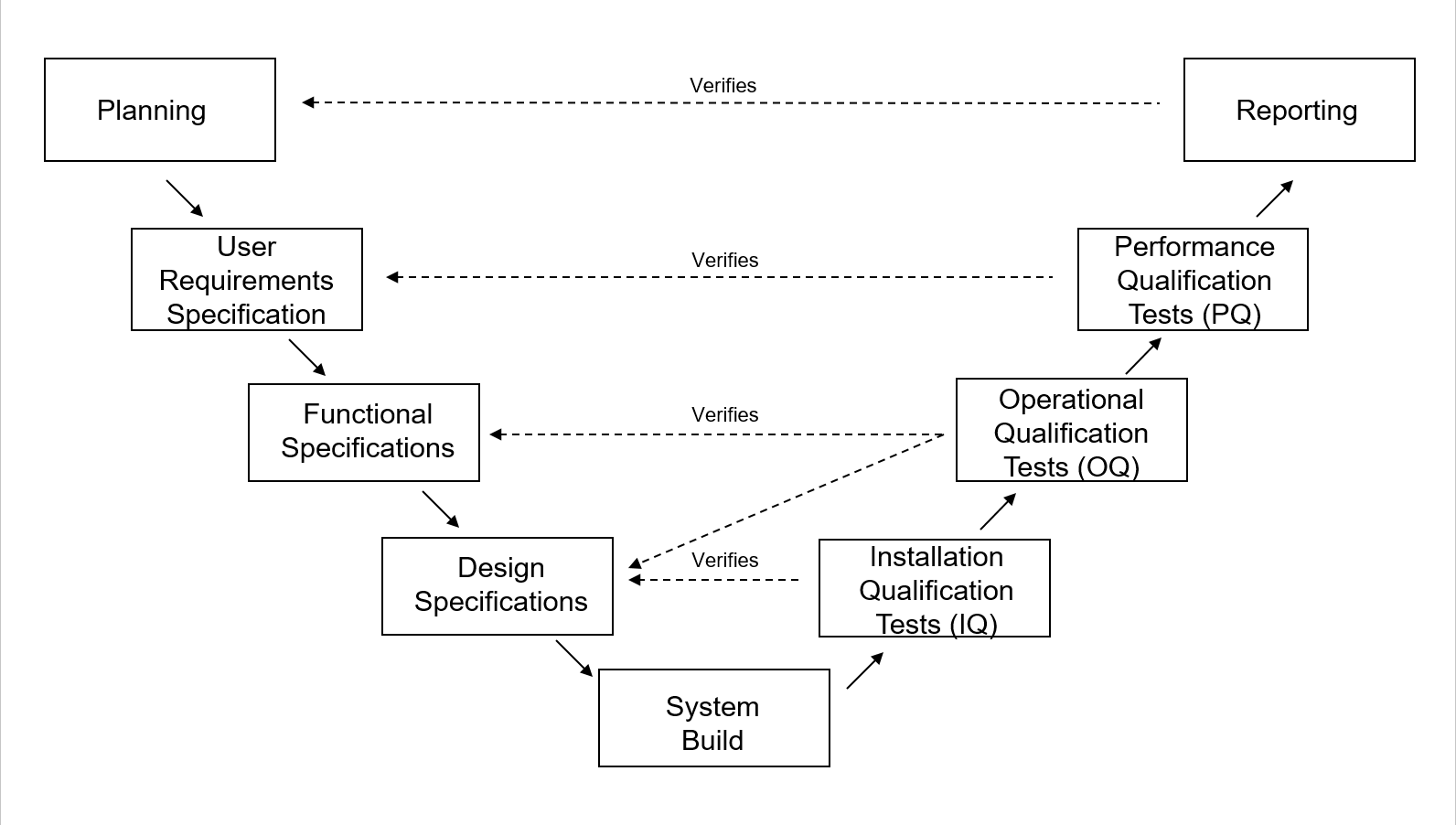
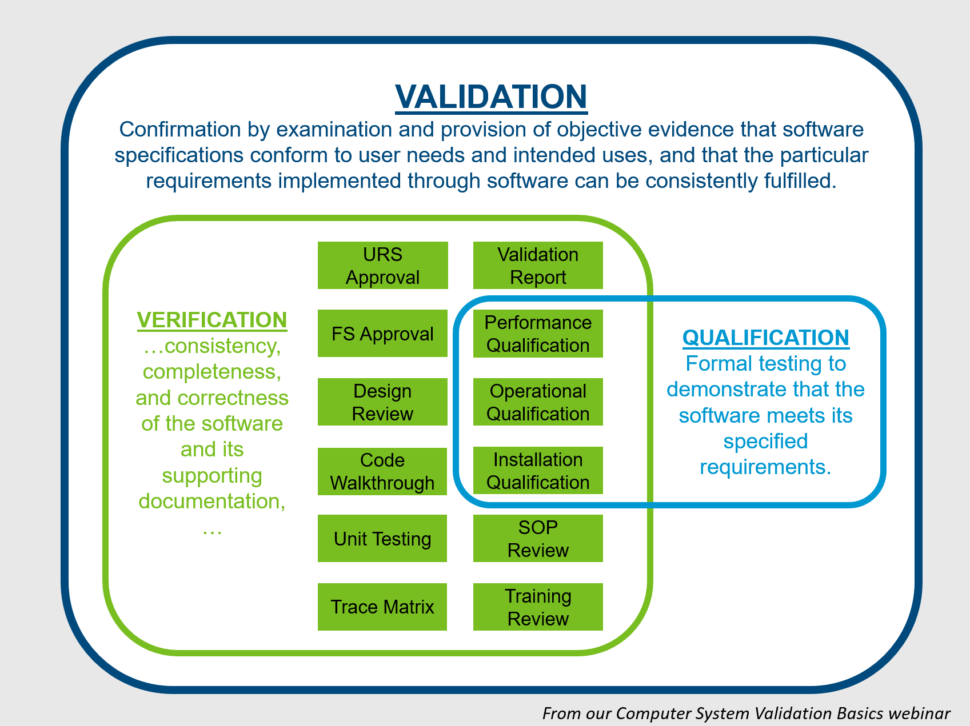
Computer System Validation (CSV) Basic Concepts, Regulations and GAMP
Computer System Validation (CSV & CSA) LearnGxP Accredited Online
Computer System Validation PowerPoint and Google Slides Template PPT
Computer System Validation Training Course (Recorded)
Computer System Validation (CSV) Validation Testing Part 1 LearnGxP
What is Computer System Validation and How Do You Do It?
Computer System Validation (CSV) Online Course And Certification
What is Computer System Validation and How Do You Do It?
Computer System Validation (CSV) Validation Testing Part 1 LearnGxP
Which Systems Do You Need To Validate?
Pharmout Has Been Training On Computer Systems Validation Since 2008, Much Has Changed Recently, Surprisingly Data Integrity Remains A Hot Topic With Regulators.
Start By Gaining Fundamental Knowledge About Computer System Validation And Maintenance Based On The Gamp 5 Second Edition.
This Course Is Designed For Validation, Quality, It, And Business Personnel Responsible For Implementing And Using Regulated Computer Systems In The Pharmaceutical, Biotech And.
Related Post: